5-HT (Serotonin) Antibodies
Serotonin Antibodies That Work
Exclusive to ImmunoStar
ImmunoStar’s 5-HT (Serotonin) antibodies were engineered by Mark Brownfield, PhD, Associate Professor, Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin, Madison. His panel of highly-regarded serotonin antibodies is exclusive to ImmunoStar and have earned a reputation among researchers as being the Serotonin antibodies that work.
Dr. Brownfield’s work on the serotonergic system and its impact on brain function has focused on the molecular anatomy of serotonergic neurons and their target neurons. His antibodies against the serotonergic system includes the antisera to serotonin precursor (5-hydroxytryptophan), primary metabolite (5-hydroxyindole acetic acid), antibodies to serotonin transporter (the target of a class of antidepressant drugs), and five serotonin receptors (5HT1a, 5HT2a, 5HT2c, 5HT5a, and 5HT7).
Dr. Brownfield’s 5HT (Serotonin) studies have shown a correlation between the serotonergic system and circuits important in neuroendocrine, autonomic, emotional, circadian, visual, and motor systems.
Publications – Search our Publications database for 5-HT Serotonin research by antibody name, number, author, species, and other keywords.
We at ImmunoStar are deeply grateful for Dr. Mark Brownfield’s generous services and contribution to the science community over the decades. Our mission is to provide the highest of quality primary antibodies and Mark did just that. It is our great pleasure to carry out Dr. Mark Brownfield’s quality antibodies and continue to share his work with the world.
“The word on the science street is that these are the only antibodies for these receptors that have worked (5HT2A #24288, 5HT2C #24505, and 5HT7 #24430).”
~Tiina Negron, Clinical Trial Coordinator and Lab Manager, Drexel University
ImmunoStar’s 5-HT (Serotonin) Antibodies
5-HIAA Antibody #24274

IHC image of neurons staining for ImmunoStar 5-H1AA antibody in the raphe nucleus of the rat brainstem.
The ImmunoStar #24274 5-HIAA (5-Hydroxyindoleacetic Acid) Antibody produces moderate labeling of raphe neurons in normal rat. In rats whose serotonergic system has been activated, staining intensity is increased to a maximum label. Recommended dilution of the antiserum is 1/4000-1/8000 for biotin-streptavidin/HRP technique.
The specificity of the antiserum was evaluated using a model system of gelatin-indole plugs by a method similar to published procedures (Schipper and Tilders, 1983). Results showed that the 5-HIAA antibody dose dependently stained 5-HIAA but did not stain any concentration of 5-HT or 5-HTP. The antiserum was also tested by pre-adsorption at 25 µg/mL with various BSA conjugates. While pre-adsorption with 5-HIAA conjugate completely eliminates immunolabeling, pre-adsorption with conjugates of 5-HT,5-HTP and dopamine had no effect on staining intensity or distribution of stain.
Photo Description: The tissue was fixed with 4% formaldehyde in phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:5000 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
5-HT 1A Receptor Antibody #24504
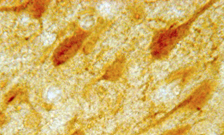
IHC image of neurons staining for the ImmunoStar 5-HT1A Receptor antibody in the rat cortex.
ImmunoStar #24504 5-HT1A Receptor Antibody: The histochemical antibody for 5-HT1A receptor is generated in a rabbit against synthetic peptide sequence corresponding to amino acids 294-312 of the rat 5-HT1A receptor. The antiserum is provided as 100 µl of affinity purified serum containing 1% BSA. Controls: The ImmunoStar 5-HT1A receptor antiserum was quality control tested using standard immunohistochemical methods.
The antiserum demonstrates strongly positive labeling of rat cortex, arcuate and hippocampus using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilutions are 1/200 – 1/300 in PBS – Bn/Av-HRP detection. Intensification methods such as nickel will approximately double the dilution factor as recommended.
The antibody was characterized by immunohistochemistry and Western blot. Western blot showed a single band of approximately 45 kD using a dilution of 1/100. The western information is included as specificity information. Preincubation of the antibody with an excess of the synthetic peptide blocked staining. Immunohistochemical staining of rat brain correlates well with Northern analysis, in situ hybridization and receptor autoradiography.
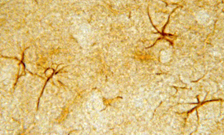
IHC image of neurons staining for the ImmunoStar 5-HT1A Receptor of astrocytes in the rat hippocampus.
BlastP database sequence homology searches confirmed that this sequence is unique to rat, mouse and human 5-HT1A receptors.
Photo Description: The tissue was fixed with 4% formaldehyde/0.05% glutaraldehyde in 0.1 M phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:500 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
5-HT 2A Receptor Antibody #24288

Low magnification IHC image of neurons staining for the ImmunoStar 5-HT2A receptor in the rat cortex.
The ImmunoStar #24288 5-HT 2A Receptor Antibody is provided as 100 uL of affinity purified serum in PBS (0.02 M sodium phosphate with 0.15 M sodium chloride, pH 7.5) with 1% BSA (bovine serum albumin), and 0.02% sodium azide. Reacts with mouse, rat, and rabbit species.
The images above labeled “ImmunoStar Rabbit 5-HT 2A receptor antibody” are the results of staining of the mouse 5-HT 2A receptor in the brain, courtesy of Dr. Magdalena Zaniewska, Max-Delbruck-Centrum fur Molekulare Medizin Berlin, Germany.

Neuronal expression of the ImmunoStar 5-HT 2A receptor in the rat amygdala.
The ImmunoStar 5HT 2A receptor antibody was quality control tested using standard immunohistochemical methods. The antiserum demonstrates strongly positive labeling of rat cortex, amygdala and hippocampus using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilutions are 1/300 – 1/500 in PBS/0.3% Triton X-100 – Bn/Av-HRP Technique . The addition of intensifying reagents such as nickel ammonium sulfate to the chromogen solution will approximately double the dilution factor as recommended.
Immunolabeling is completely abolished by preadsorption with synthetic rat 5HT2A receptor (22-41). Immunolabeling of Western blot revealed a single band of approximately 53kD.

ImmunoStar 5-HT (Serotonin) 2A Receptor Antibody photo of the rat cortex
Photo Description: The tissue was fixed with 4% formaldehyde in 0.1 M phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:500 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
5-HT 2C Receptor Antibody #24505

Gray scale IHC image of coronal rat brain section staining for the ImmunoStar 5-HT2C receptor.
The ImmunoStar #24505 5-HT2C Receptor antiserum was quality control tested using standard immunohistochemical methods. The antiserum demonstrates strongly positive labeling of rat choroid plexus and hippocampus using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilutions are /500 – 1/1000 in PBS – Bn/Av-HRP technique. Intensification methods such as nickel will approximately double the dilution factor as recommended.
The antibody was characterized by immunohistochemistry and Western blot. Western blotting revealed a single band of approximately 70 kD.

High magnification image of ImmunoStar 5-HT 2C receptor expression in the cortex of the rat brain.
Preincubation of the antibody with an excess of the synthetic peptide blocked staining. Immunohistochemical staining of rat brain correlates well with Northern analysis, in situ hybridization and receptor autoradiography. BlastP database sequence homology searches confirmed that this sequence is unique to rat, mouse and human 5-HT2C receptors.
High magnification image of ImmunoStar 5-HT 2C receptor expression in the cortex of the rat brain.
Photo Description: The tissue was fixed with 4% formaldehyde/0.05% glutaraldehyde in 0.1 M phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:1000 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
5-HT 5A Receptor Antibody #24429

IHC image of neurons staining for the ImmunoStar 5-HT5A receptor in the rat hippocampus.
The ImmunoStar #24429 5-HT5A Receptor Antibody was quality control tested using standard immunohistochemical methods. The antiserum demonstrates strongly positive labeling of rat cortex and hippocampus using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilution is 1/200 – 1/400 in PBS/0.3% Triton X-100 – Bn/Av-HRP. Intensification methods such as nickel will approximately double the dilution factor as recommended.
The antibody was characterized by immunoblotting and immunohistochemistry. Immunoblots of rat brain extracts revealed the presence of two bands at molecular weights of 41 and 47 kD. The lower weight band agrees with the calculated molecular weight based on amino acid sequence. The higher weight may represent glycosylated receptor protein.
Immunohistochemical staining of rat brain correlates well with Northern blot analysis and in situ hybridization studies. Immunolabeling is completely abolished by preadsorption with synthetic rat 5-HT5A receptor (17-34). BlastP database sequence homology searches indicate that the amino acid sequence is unique to rat and mouse 5-HT5A receptor.
IHC image of neurons staining for the ImmunoStar 5-HT5A receptor in the rat cortex.

IHC image of neurons staining for the ImmunoStar 5-HT5A receptor in the rat cortex.
Photo Description: The tissue was fixed with 4% formaldehyde/0.05% glutaraldehyde in 0.1 M phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:500 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
5-HT 6 Receptor Antibody #24507
 The ImmunoStar #24507 5-HT6 Receptor antiserum was quality control tested using standard immunohistochemical methods. The antiserum demonstrates significant labeling of rat cortex, amygdala and hippocampus and other areas using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilutions are /500 – 1/1000 in PBS – Bn/Av-HRP technique. Intensification methods such as nickel will approximately double the dilution factor as recommended.
The ImmunoStar #24507 5-HT6 Receptor antiserum was quality control tested using standard immunohistochemical methods. The antiserum demonstrates significant labeling of rat cortex, amygdala and hippocampus and other areas using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilutions are /500 – 1/1000 in PBS – Bn/Av-HRP technique. Intensification methods such as nickel will approximately double the dilution factor as recommended.
The antibody was characterized by immunohistochemistry and Western blot. Western blotting revealed a single band of approximately 53 kD.
Preincubation of the antibody with an excess of the synthetic peptide blocked staining. Immunohistochemical staining of rat brain correlates well with Northern analysis, in situ hybridization and receptor autoradiography.
5-HT 7 Receptor Antibody #24430

IHC image of neurons staining for the ImmunoStar 5-HT7 receptor in the rat brain cortex.
The ImmunoStar #24430 5-HT7 Receptor antiserum was quality control tested using standard immunohistochemical methods. The antiserum demonstrates strongly positive labeling of rat cortex and hippocampus using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilution is 1/100 – 1/300 in PBS – biotin/avidin-HRP Technique. Note: use of Triton X-100 or other detergents is not recommended.
The antibody was characterized by immunohistochemistry. Immunohistochemical staining of rat brain correlates well with Northern blot analysis, in situ hybridization and receptor autoradiography studies. Immunolabeling is completely abolished by preadsorption with synthetic rat 5-HT7 receptor (8-23). BlastP database sequence homology searches indicate that the amino acid sequence is unique to rat 5-HT7A, 5-HT7B and 5-HT7C. There is also significant sequence overlap with the mouse and human forms.
Photo Description: IHC image of neurons staining for the 5-HT7 receptor in the rat brain cortex. The tissue was fixed with 4% formaldehyde/0.05% glutaraldehyde in 0.1 M phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:500 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
5-HT Transporter Antibody #24330

IHC image of neurons staining for the ImmunoStar 5-HT transporter in the raphe nucleus of the rat brainstem.
The ImmunoStar #24330 5HT (Serotonin) Transporter Antibody was quality control tested using standard immunohistochemical methods. The antiserum demonstrates strongly positive labeling of rat raphe nuclei, hypothalamus, cortex and spinal cord using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilution is 1/10,000 – 1/15,000 in PBS/0.3% Triton X-100 – Biotin/avidin-HRP.
By Western blot analysis using rat brain extracts of cortex, hypothalamus, midbrain, and hindbrain, the antibody specifically labels a single band. The predicted molecular weight of serotonin transporter based on its nucleic acid sequence is 67,815. The actual weight that one might observe from tissue extracts could be higher due to glycosylation. We validated the antibody with rat SERT overexpressed in an insect cell expression system. The MW from that was about 67 kD. Our SERT antibody dilution was 1:20,000 using chemiluminescent detection.
Immunolabeling is completely abolished by pre-adsorption with synthetic rat 5HT transporter (602-622).
ImmunoStar 5-HT (Serotonin) Transporter Antibody florescent secondary.

ImmunoStar 5-HT (Serotonin) Transporter Antibody florescent secondary.
Photo Description: The tissue was fixed with 4% formaldehyde in 0.1 M phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:10000 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
5-HTP Antibody #24446

IHC image of neurons staining for ImmunoStar 5-HTP in the raphe nucleus of the rat brainstem.
The ImmunoStar #24446 5 HTP Antibody has a proven maximum biotin-streptavidin/HRP staining at a 1/1000 – 1/2000 dilution in rat raphe nuclei. Optimal dilution will vary depending upon fixation, labeling technique and/or detection system; therefore, a dilution series is recommended.
The specificity of the antiserum was evaluated using a model system of gelatin-indole plugs by a method similar to published procedures (Shipper and Tilders, 1983). Results showed that the 5-HTP antibody dose dependently stained 5-HTP but did not stain any concentration of 5-HT or 5-HIAA.
The antiserum was also tested by pre-adsorption with indole/paraformaldehyde/BSA conjugates. Staining was completely blocked by pre-adsorption with 5-HTP conjugate and unaffected by 5-HIAA or 5-HT conjugate.
Photo Description: IHC image of neurons staining for 5-HTP in the raphe nucleus of the rat brainstem. The tissue was fixed with 4% formaldehyde in phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% goat serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:2000 for 48 hours, goat anti-rabbit secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
